Does Pure Silver Turn Black? The Ultimate Care Guide
Let’s cut to the chase: yes, pure silver does turn black. It just happens much, much more slowly than you’d see with other silver alloys. This discolouration isn't a flaw or a sign of poor quality; it's a completely natural chemical process called tarnishing.
Think of it like an apple slice browning after you’ve left it on the counter. It's just a surface reaction to the air around it.
Why Your Silver Turns Black
So, what’s actually happening? Tarnish is the result of a slow-motion chemical dance between silver and sulphur compounds that are all around us. These tiny particles are in the air we breathe, coming from things like air pollution, humidity, and even some foods.
When silver atoms come into contact with these sulphur compounds, they react to form silver sulphide. This creates that familiar dark, filmy layer on the surface of your silver piece. The good news? This reaction is only skin-deep. It doesn't damage the precious metal underneath and, with a bit of care, it can be cleaned right off.
The key thing to understand is that the purity of the silver has a huge impact on how fast this all happens.
- Sterling Silver (92.5%): This is the most common alloy you'll find. It contains 7.5% other metals, usually copper. Copper is highly reactive and acts like a catalyst, dramatically speeding up the tarnishing process.
- Pure Silver (99.9%): Often called "fine silver," this type lacks those reactive alloy metals like copper, making it far more resistant to turning black.
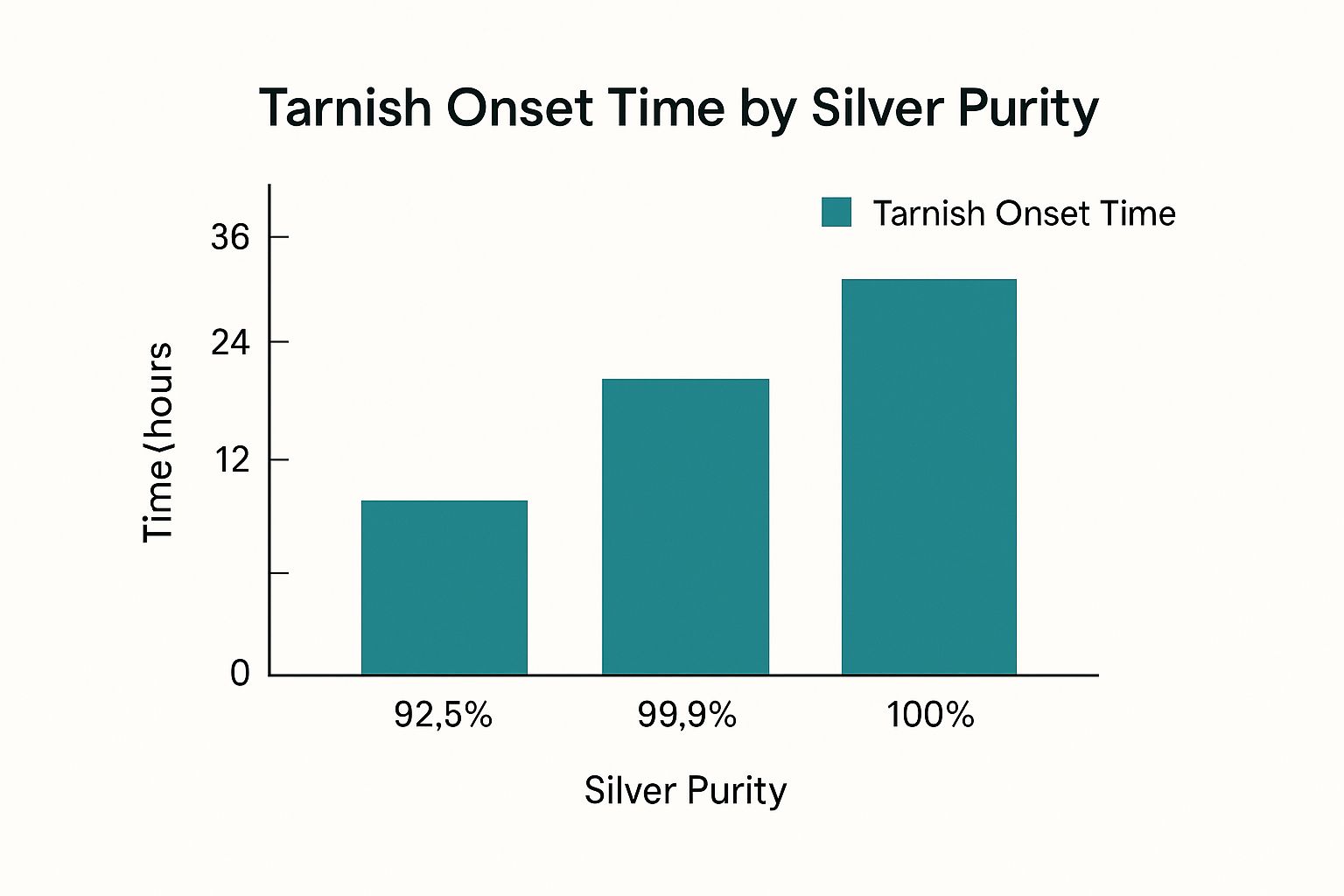
To make this distinction crystal clear, here’s a quick side-by-side comparison.
Pure Silver vs Sterling Silver Tarnish at a Glance
| Characteristic | Pure Silver (99.9%) | Sterling Silver (92.5%) |
|---|---|---|
| Composition | Almost entirely pure silver with trace impurities. | 92.5% silver, 7.5% alloy metals (usually copper). |
| Tarnish Speed | Very slow; highly resistant to tarnishing. | Much faster; the copper content accelerates the reaction. |
| Durability | Softer and more prone to scratches and dents. | Harder and more durable, making it ideal for jewellery. |
Ultimately, while pure silver is much more tarnish-resistant, its softness makes it less practical for everyday items. Sterling silver's blend of durability and beauty is why it remains the industry standard, even if it means a little more polishing now and then.
The Science Behind Why Silver Tarnishes

If you've ever noticed your silver jewellery or cutlery developing a dark, dull film, you're not seeing dirt or rust. What you're witnessing is tarnish—a simple, and surprisingly interesting, chemical makeover happening right on the surface. It's a natural response to the environment, much like how a bronze statue develops that classic green patina over time. In a way, it’s a sign you’re dealing with the real deal.
The main culprit behind this transformation is hydrogen sulphide, a common gas floating around in the air. It’s released from everyday sources like air pollution, natural gas, and even certain foods—think eggs, onions, and garlic. When your silver comes into contact with these airborne sulphur compounds, a slow and silent reaction kicks off.
The Chemical Reaction Explained
This process is a form of corrosion, but it's nothing like the destructive rust that eats away at iron. Silver tarnish is purely a surface-level issue. The silver atoms on the outermost layer react and bond with sulphur, creating a brand-new compound: silver sulphide (Ag₂S). This is the dark, blackish film you see.
Think of it like your silver putting on a very thin, dark 'coat'. This layer is only skin-deep and doesn't damage the precious metal underneath. In fact, it's a testament to the silver's authenticity.
The good news is that because this is just a surface change, the silver underneath remains perfectly intact. This is exactly why a bit of polish and elbow grease can restore it to its original shine.
Why Purity Matters in Tarnishing
So, we know that silver sulphide is the chemical cause of that dark layer. But the speed at which it forms depends heavily on what else is mixed in with your silver. This is where the difference between pure silver and sterling silver really comes into play.
Pure silver is actually quite resistant to tarnishing on its own. The problem starts with the other metals mixed in to make it stronger. Sterling silver, for example, contains copper, and this copper acts as a catalyst, dramatically speeding up the reaction with those sulphur compounds in the air. If you want to dive deeper into the chemistry, you can discover insights into why different silver types tarnish at varying rates.
This is why your everyday sterling silver ring might start looking dull much faster than, say, a pure silver heirloom that spends most of its life in a display case. Understanding this difference is key to knowing how to care for your specific silver items.
Why Sterling Silver Is a Tarnish Magnet
If pure silver is so resistant to tarnish, you might be wondering why that favourite sterling silver piece of yours seems to turn dark so quickly. The truth is, the problem isn't the silver itself—it's the company it keeps. Sterling silver isn't pure metal; it's an alloy, a deliberate mixture of metals created for a very practical reason.
This go-to material for quality jewellery is 92.5% pure silver blended with 7.5% other metals, almost always copper. You can get the full story on its make-up in our guide to what 925 sterling silver is. While beautiful, pure silver is incredibly soft. It’s simply not tough enough for pieces like rings or bracelets that have to stand up to the knocks and bumps of daily life. Adding a bit of copper is what gives it the necessary hardness and durability.
The Real Culprit: Copper
But that added strength comes with a chemical trade-off. It turns out that copper is far more reactive to the sulphur compounds floating around in our air than silver is. You could think of the copper as a troublemaker in the alloy, eagerly reacting with sulphur and essentially kickstarting the tarnish process.
It’s the copper in sterling silver that acts as a catalyst, dramatically speeding up the very chemical reaction that causes tarnish. This is the main reason a sterling silver necklace can start to discolour in just a few weeks, while a pure silver heirloom might stay bright for years under the exact same conditions.
This isn't a new discovery, of course. Artisans have understood this for centuries. In India, for example, silversmiths have long known that "fine silver," often marked as 999 silver (meaning 99.9% pure), is much less likely to blacken. It's why they often prefer it for ceremonial items that need to maintain their lustre. The choice is ultimately a practical one: with sterling silver, you get everyday durability in exchange for a bit more polishing now and then.
How Everyday Life Can Accelerate Tarnish

Your silver jewellery doesn't live in a bubble. The world around it is full of things that can speed up the tarnishing process. Think of your daily environment as being packed with "tarnish accelerators"—commonplace factors that give that natural chemical reaction a serious boost.
One of the biggest culprits? Humidity. Moisture in the air is like a superhighway for sulphur compounds, helping them find and bond to your silver's surface much faster. It's why you'll often see your favourite pieces looking duller during a humid monsoon season compared to the dry winter air.
Common Tarnish Triggers in Your Home
Beyond the general air quality, a lot of things we use every day can spell trouble for silver. Your daily routine might be unintentionally putting your jewellery in the line of fire. Simply being aware of these triggers is the first step towards keeping your silver bright and beautiful.
Here are a few of the most common household culprits to watch out for:
- Beauty Products: Things like lotions, perfumes, hairsprays, and cosmetics leave behind chemical residues that react with silver. A good rule of thumb is to make your jewellery the very last thing you put on.
- Household Chemicals: Be careful with chlorine bleach, ammonia, and even the chlorinated water from your tap. These are incredibly harsh and can cause almost instant discolouration.
- Certain Materials: Surprisingly, materials like wool, felt, and even rubber bands can release sulphur compounds over time. This makes them poor choices for long-term storage containers.
- Sulphur-Rich Foods: If you've been handling foods like eggs, onions, garlic, or mayonnaise, be sure to wash your hands before touching your silver. You can easily transfer sulphur right onto the metal.
The environment's impact is huge. You can really see this in how differently pure silver and sterling silver react to polluted air. For example, in a city with high pollution, a pure silver item might start showing tarnish after 6 to 12 months, but a piece of sterling silver jewellery could start turning in just 1 to 2 months.
It’s so important to understand the nuances between different types of silver. You can learn more about the properties of 925 silver in our detailed guide. This kind of knowledge helps explain why sterling silver, even though it's more durable, needs a bit more care. This real-world difference in tarnishing speed is a perfect example of chemistry in action, and these detailed findings on atmospheric corrosion show just how much the air around us matters.
How to Safely Clean and Restore Your Silver

Bringing your silver back to its brilliant best is a lot simpler than you might think. The real secret is to start gently and only move on to stronger methods if the tarnish puts up a fight.
For most day-to-day upkeep, a simple microfibre cloth or a proper silver polishing cloth is all you need. A quick wipe-down after wearing your pieces can work wonders, removing skin oils and everyday grime before they have a chance to kickstart the tarnishing process. If you want to dive deeper into the best materials for your jewellery, our complete guide at https://thespringstory.com/blogs/925-silver has you covered.
When you're faced with more stubborn discolouration, a homemade remedy is often the perfect solution.
Gentle Cleaning With a Baking Soda Paste
You can easily whip up a mild paste that lifts away tarnish without scratching the delicate surface of your silver. It’s surprisingly effective.
- Make the Paste: Just mix a little bit of baking soda with a few drops of water. You're aiming for a smooth consistency, almost like toothpaste.
- Apply Gently: Using a soft cloth or even just your fingertips, gently rub this paste onto the tarnished spots.
- Rinse and Dry: Once you’re done, rinse the piece thoroughly under warm water. The final step is crucial: dry it completely with a clean, soft cloth to avoid any water spots.
Remember, the goal here is to clean, not to scrub. Being too aggressive can leave fine scratches on the silver, which dulls its shine over time. A soft touch is always best.
What to Avoid When Cleaning Silver
Knowing what not to do is just as important as knowing the right way to clean your silver. Some everyday household products can cause serious, sometimes irreversible, damage.
Be sure to steer clear of these:
- Abrasive Materials: Anything gritty, like certain toothpastes or scouring pads, will scratch the surface.
- Harsh Chemicals: Never let your silver come into contact with bleach, ammonia, or even chlorinated water from a swimming pool. These can cause severe discolouration and pitting.
While we're talking about pure and sterling silver here, many of these gentle methods work for silver-plated items too. For specific advice on that, this guide on how to clean silver plate silverware is a fantastic resource. By sticking to these safe, proven techniques, you can keep your silver looking incredible for years to come.
Smart Storage Habits to Prevent Tarnish
When it comes to tarnish, an ounce of prevention is truly worth a pound of cure. The best way to deal with that frustrating discolouration is to stop it from ever getting a foothold. It all comes down to smart storage.
Think about what tarnish needs to thrive: air and moisture. If you can cut off its supply to those two things, you dramatically slow down the chemical reaction. This simple, proactive approach means you'll spend far less time polishing and a lot more time admiring your silver's brilliant shine.
Create an Anti-Tarnish Environment
Your first move should be to store each piece of silver jewellery separately. This has a double benefit—it stops items from scratching each other and it quarantines any tarnish that might start to form on one piece. An airtight container is your best ally in this fight.
The golden rule for silver storage is simple: keep it in a cool, dry, and dark place. Humidity is the number one enemy, so keeping your silver off a damp bathroom windowsill will make a world of difference.
Ready to take your storage game to the next level? Here are a few fantastic tools to create a fortress against tarnish:
- Anti-tarnish pouches or cloths: These aren't just any old fabric. They're specially treated to soak up the airborne sulphur compounds that turn your beautiful silver black.
- Airtight plastic bags: You don't need to get fancy. A humble zip-lock bag can work wonders. Just pop your jewellery inside and squeeze out as much air as possible before sealing it.
- Silica gel packets: You know those little white packets you find in new shoe boxes or vitamin bottles? Don't throw them out! Tossing one into your jewellery box is a great way to absorb excess moisture.
- Anti-tarnish strips: These little paper strips are like magnets for pollutants. They actively pull the harmful compounds out of the air, drawing them away from your precious silver.
By making these small habits part of your routine, you create a safe haven for your silver, keeping it looking its best for years to come.
Still Have Questions About Silver Tarnish?
Even when you know the science behind why silver tarnishes, a few practical questions always seem to come up. Let's dig into a couple of the most common ones I hear.
Does Wearing My Silver Actually Help Prevent Tarnish?
It can, surprisingly! The natural oils on your skin often create a sort of protective layer, slowing down the chemical reaction that causes tarnish. So, wearing your favourite pieces regularly can genuinely help keep them bright.
However, it's not a foolproof plan. If your skin is more on the acidic side or if you sweat a lot, this can actually accelerate the tarnishing process. It really comes down to your individual body chemistry.
The best habit to get into? Give your jewellery a quick, gentle wipe with a soft cloth after you take it off for the day.
Are Those Chemical Silver Dips a Good Idea?
I'd advise you to be very, very careful with these. Chemical dips work fast, that's for sure, but they are incredibly harsh and can easily cause more harm than good.
They're so aggressive that they can strip away the lovely, intentional darkening (called patina) that gives antique pieces their character and depth. Worse, they can permanently damage porous or delicate gemstones like pearls, opals, and turquoise.
Always start with the gentlest cleaning method and only escalate if you have to. A simple polishing cloth or a homemade paste of baking soda and water should be your first port of call, long before you even think about reaching for a chemical dip.



